Fully Configurable
em-PACT is configured to mirror your study activation process including:
Multiple Study Types
Configure study activation workflows and tasks based on the type of study being initiated. Some examples include:
- Investigator Initiated Trial
- Industry Sponsored Trial
- Nationally Sponsored Trial
- Externally Peer-Review Trial
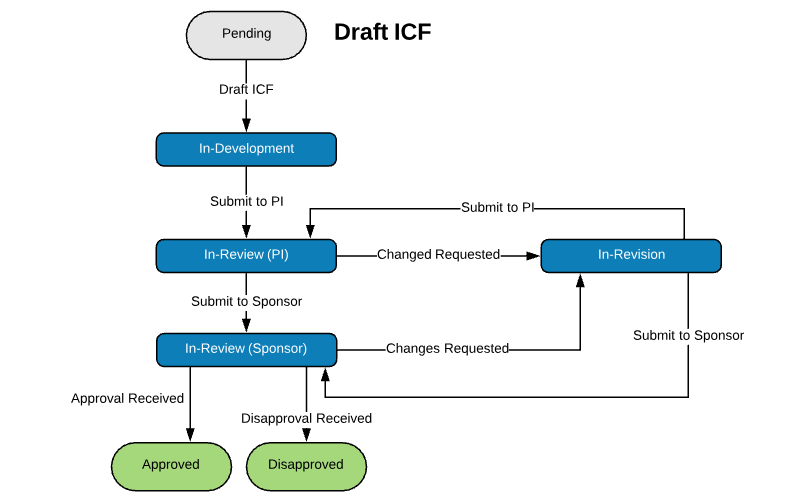
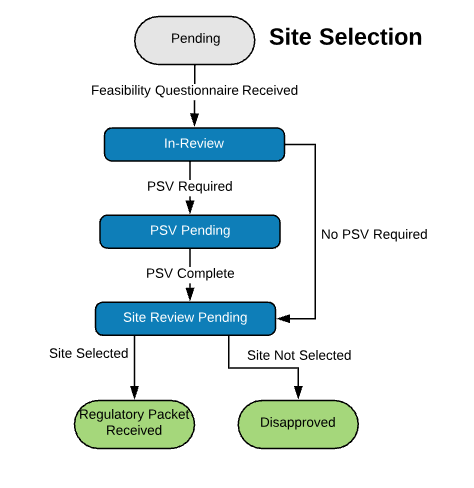
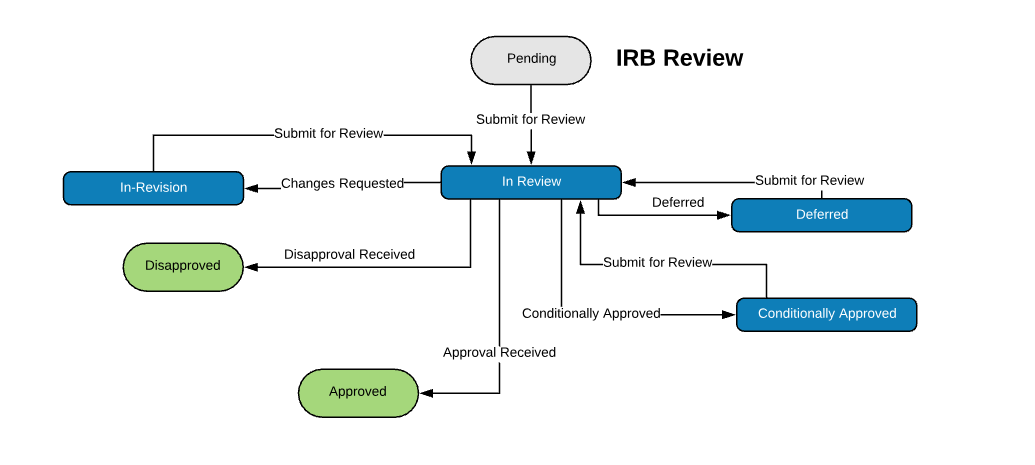
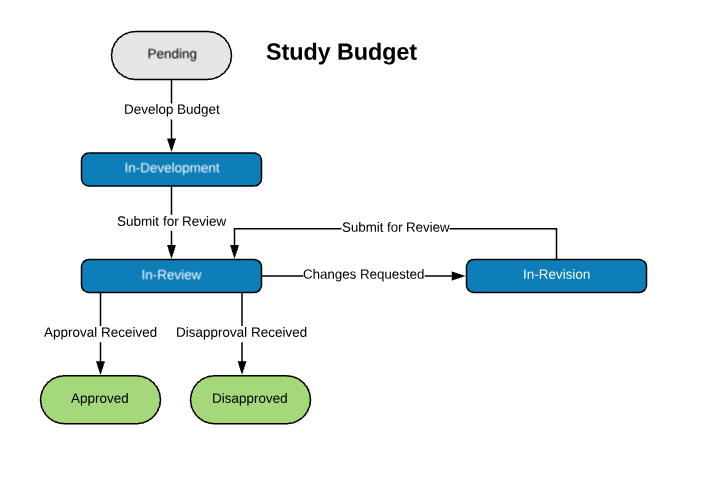
Extensible Library of Study Activation Tasks
Start with a standard library of study activation tasks and add/modify as needed. For example:
| Concept Development | Develop Letter of Intent (LOI) |
| LOI – Sponsor Review | |
| Protocol Development | Draft Protocol |
| Protocol – Sponsor Review | |
| Protocol – Feasibility Review | |
| Industry Trials | Execute Confidentiality Disclosure Agreement (CDA) |
| Site Selection | |
| Regulatory Packet Assembly | Create Informed Consent Forms (ICFs) |
| Complete Financial Disclosure Forms (FDFs) | |
| Study Activation | Scientific Protocol Review |
| Institutional Review Board (IRB) Review | |
| Study Billing Grid | |
| Study Budget | |
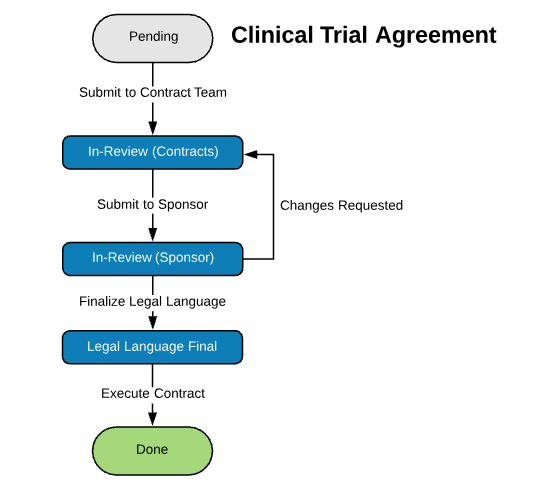
| Clinical Trial Agreement (CTA) | |
| Site Initiation Visit |
Flexible Workflows
Start with a standard library of task workflows and add/modify as needed: